Rewriting the Rules of Engagement: AI’s Role in the Future of Medical Communication
In today’s fast-paced medical landscape, healthcare providers and pharmaceutical companies face mounting pressure to deliver accurate, compliant, and timely responses to medical inquiries. Traditional methods, however, often struggle with inefficiencies, human error, and regulatory complexity. Enter Artificial Intelligence (AI)—a game-changer for medical information management.
At SciMax Global, we’ve harnessed AI to supercharge our SCIMAX MI platform, transforming how medical affairs teams operate. Below, we explore how AI bridges the gap between speed and compliance while keeping users firmly in control.
Why AI Matters in Medical Information Management
AI isn’t about replacing humans—it’s about empowering them. By automating repetitive tasks, analyzing vast datasets, and flagging risks, AI allows medical teams to focus on high-value decisions. Here’s how it adds value:
- Faster Responses: AI cuts through data clutter to recommend pre-approved answers.
- Enhanced Compliance: Automatically detect adverse events (AEs) and safeguard sensitive data.
- Smarter Workflows: From email triage to document summarization, AI handles the grunt work.
But the key lies in balance. While AI accelerates processes, human expertise remains irreplaceable for nuanced judgments (e.g., off-label inquiries). At SciMax, we design AI to assist, not replacement users always have the final say.
The Role of AI in Medical Information Management
Enhancements through AI:
- AI improves decision-making by analysing large datasets, identifying patterns, and providing actionable insights. For example, AI can recommend optimal responses to medical inquiries based on historical data.
- AI automates repetitive tasks like case intake, PII redaction, and quality assurance, reducing manual errors and improving efficiency.
- AI ensures adherence to evolving healthcare regulations by detecting compliance risks, auto-redacting sensitive data, and monitoring regulatory updates.
Balancing AI Assistance vs. Automation Risks:
- Fully automated systems risk errors that could harm patients or compromise data privacy. A balanced approach integrates AI as a decision-support tool while preserving human oversight to validate outcomes.
- User control mechanisms (e.g., manual overrides) are essential for critical tasks like PII redaction and adverse event identification to mitigate risks of over-reliance on automation.
Challenges and Regulatory Considerations:
- Ensuring high-quality data while protecting patient privacy is crucial. Governments must provide infrastructure for secure data sharing and compliance monitoring.
- Constantly evolving regulations require AI systems capable of real-time adaptation to new standards.
- Ethical concerns include transparency in AI decision-making processes and the potential for bias in algorithms.
AI Use Cases in SciMax Global’s SCIMAX MI Platform
SCIMAX MI platform leverages AI-driven solutions to address specific challenges in medical information management. Below is a structured analysis of key use cases:
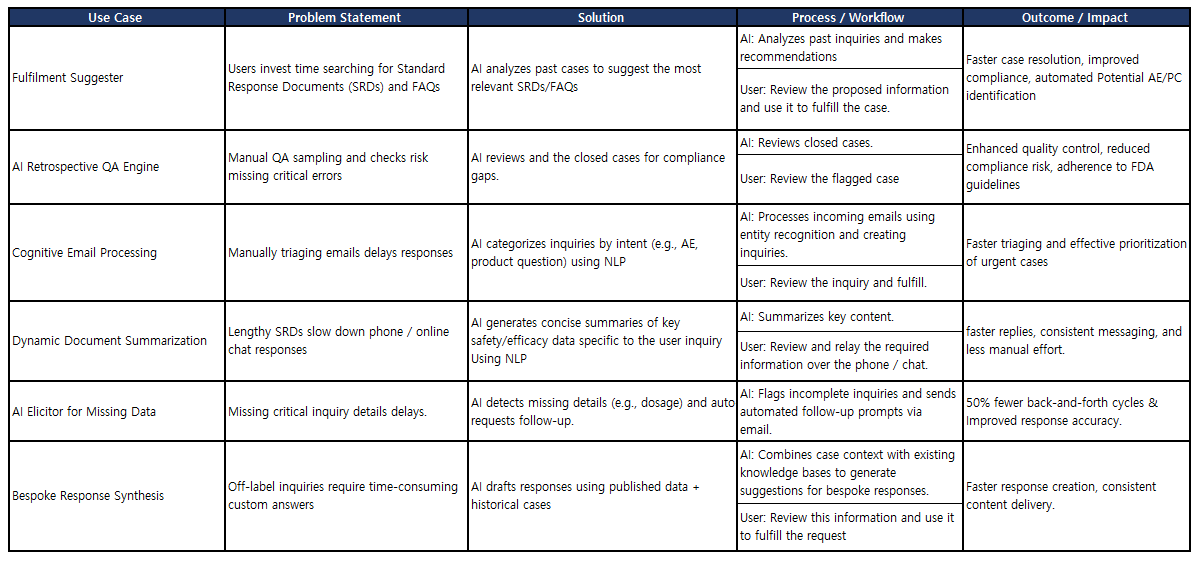
| Use Case | Problem Statement | Solution | Process / Workflow | Outcome / Impact |
|---|---|---|---|---|
| Fulfilment Suggester | Users invest time searching for Standard Response Documents (SRDs) and FAQs | AI analyzes past cases to suggest the most relevant SRDs/FAQs | AI: Analyzes past inquiries and makes recommendations User: Review the proposed information and use it to fulfill the case. |
Faster case resolution, improved compliance, automated Potential AE/PC identification |
| AI Retrospective QA Engine | Manual QA sampling and checks risk missing critical errors | AI reviews and the closed cases for compliance gaps. | AI: Reviews closed cases. User: Review the flagged case |
Enhanced quality control, reduced compliance risk, adherence to FDA guidelines |
| Cognitive Email Processing | Manually triaging emails delays responses | AI categorizes inquiries by intent (e.g., AE, product question) using NLP | AI: Processes incoming emails using entity recognition and creating inquiries. User: Review the inquiry and fulfill. |
Faster triaging and effective prioritization of urgent cases |
| Dynamic Document Summarization | Lengthy SRDs slow down phone / online chat responses | AI generates concise summaries of key safety/efficacy data specific to the user inquiry Using NLP | AI: Summarizes key content. User: Review and relay the required information over the phone / chat. |
Faster replies, consistent messaging, and less manual effort. |
| AI Elicitor for Missing Data | Missing critical inquiry details delays. | AI detects missing details (e.g., dosage) and auto requests follow-up. | AI: Flags incomplete inquiries and sends automated follow-up prompts via email. | 50% fewer back-and-forth cycles & Improved response accuracy. |
| Bespoke Response Synthesis | Off-label inquiries require time-consuming custom answers | AI drafts responses using published data + historical cases | AI: Combines case context with existing knowledge bases to generate suggestions for bespoke responses. User: Review this information and use it to fulfill the request |
Faster response creation, consistent content delivery. |
Striking the Right Balance: AI + Human Expertise
While AI drives efficiency, human oversight ensures safety and compliance. For example:
- High-Risk Decisions: Users validate AI-generated responses for off-label inquiries.
- Transparency: Explainable AI (XAI) shows why a recommendation was made (e.g., data sources).
- Customization: Users adjust AI sensitivity (e.g., strict vs. lenient PII detection).
At SciMax, we call this the “Human-in-the-Loop” (HITL) approach—blending AI’s speed with human judgment.
Conclusion: Embrace AI, Empower Teams
AI isn’t just a buzzword—it’s a strategic tool to future-proof medical information management. By automating repetitive tasks and surfacing insights, SciMax’ s SCIMAX MI platform lets teams focus on what matters: improving patient outcomes.
Ready to transform your workflows?
Contact us to explore how AI can elevate your medical affairs operations.
Author
Sainath Racherla
Associate Director – Program Management

