Optimize and Streamline Publication Management for Global Scientific and Medical Affairs
SCIMAX Pubs is a comprehensive publication planning, execution, and monitoring solution capable of optimizing and streamlining the scientific publication lifecycle. SCIMAX Pubs provides global transparency and consistent management of a publication program and related projects to help ensure compliant and consistent dissemination of scientific information. The user-friendly, easy to implement, web-based solution is highly configurable so that the current and future publication needs of individual scientific teams and the overall organization are met with ease.
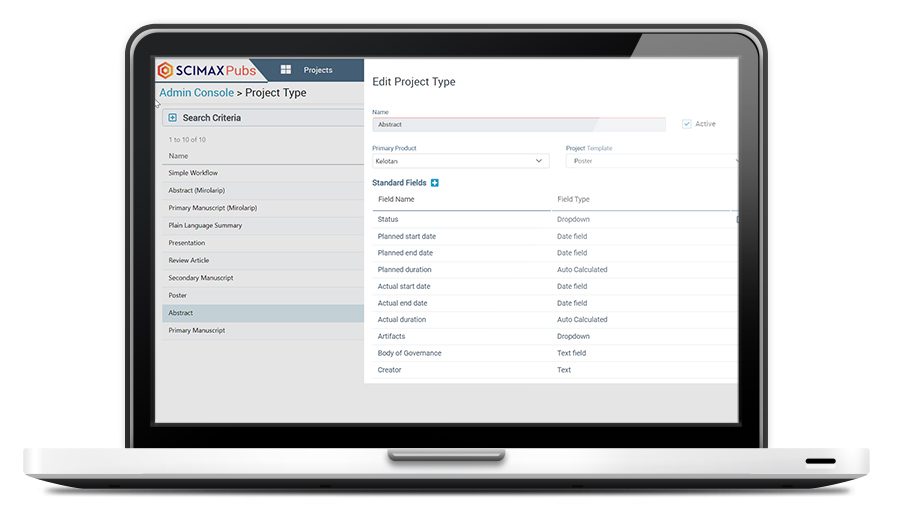

Ensure compliance and provide flexibility when setting up and managing a publication project.
Set-up milestones, tasks, and timelines specific to project types, products, regions, disease states, and more.
Delete and add milestones and tasks at the project level based on permissions.
Create multiple project templates with pre-defined milestones and tasks which align with standard operating procedures (SOPs) and Good Publication Practices.
Access full audit trail which captures review and approval history.
Efficiently manage publication projects and reduce timelines.
Collaborate on the authoring and reviewing of documents with track changes and comments.
Reconcile comments and changes from authors and approvers in a single document version.
Accept, delegate, decline, and reassign tasks with different permission settings.
Receive notifications of assigned tasks with automated system alerts and emails.

See Scimax MI in Action -
Request a demo today

Measure department value by accessing real-time dashboards and views that capture overall strategic objectives, operational tasks, and Key Performance Indicators (KPIs).
Manage overall workload according to deadlines and priorities using queues.
Access project timelines and status in different views including Gantt charts.
Utilize prebuilt and self-service dashboards to visualize reports and gain insights into publications and operational efficiency.
Track KPI such as time to submission and approval rate in real-time.
Schedule time-based reports to be delivered via email to a designated team.
Organize and centralize content and email correspondence to further reduce publication timelines.
Centralize content to avoid duplication and ensure article accuracy and data integrity.
Document version control with a history of all redline and draft versions.
Manage all email communications between external users and your company directly within SCIMAX Pubs.


Client-controlled administration allows super-users to manage and change the system as needed.
Manage and update workflows, timelines, milestones, and tasks for all project types.
Add custom fields, new email templates, and dropdown values.
Create security policies for user access at the product or project level.
Configure alerts and notifications based on current needs.
Connect to data in other solutions to gain additional efficiency and more insight.
Connect existing content management, clinical trial management, and finance systems with available API-based integrations.
Assist publication managers in selecting the most appropriate journal or congress for submission with the built-in Journal and Congress Repository (JCR).



