SciMax Global – 2026 Product Update
Chief Product Officer Briefing
Medical Affairs is undergoing a global shift from reactive information delivery to proactive, data-driven scientific leadership. With this transformation comes the need for technology that elevates scientific rigor, amplifies operational impact, and upholds compliance without slowing teams down.
We have been listening. And we have been building.
We are excited to announce a major expansion of our AI agent platform SCIMAX ARIN, a suite of autonomous, intelligent agents designed specifically for the pharmaceutical industry’s medical affairs most critical workflows.
We have identified 10 strategic agents addressing the highest-priority scientific and operational use cases. We have 2 agents currently in active development, 4 agents in sandbox for early client’s access, and we’re officially releasing 4 production-ready agents in 2026.
This isn’t just a feature release. This is the beginning of a fundamental reshaping of how medical affairs teams’ work.
Why Now? The Market Inflection
The timing couldn’t be clearer. The global AI agents’ market is experiencing explosive growth projected to expand at a 45.82% CAGR through 2034, reaching $236 billion in total market value.
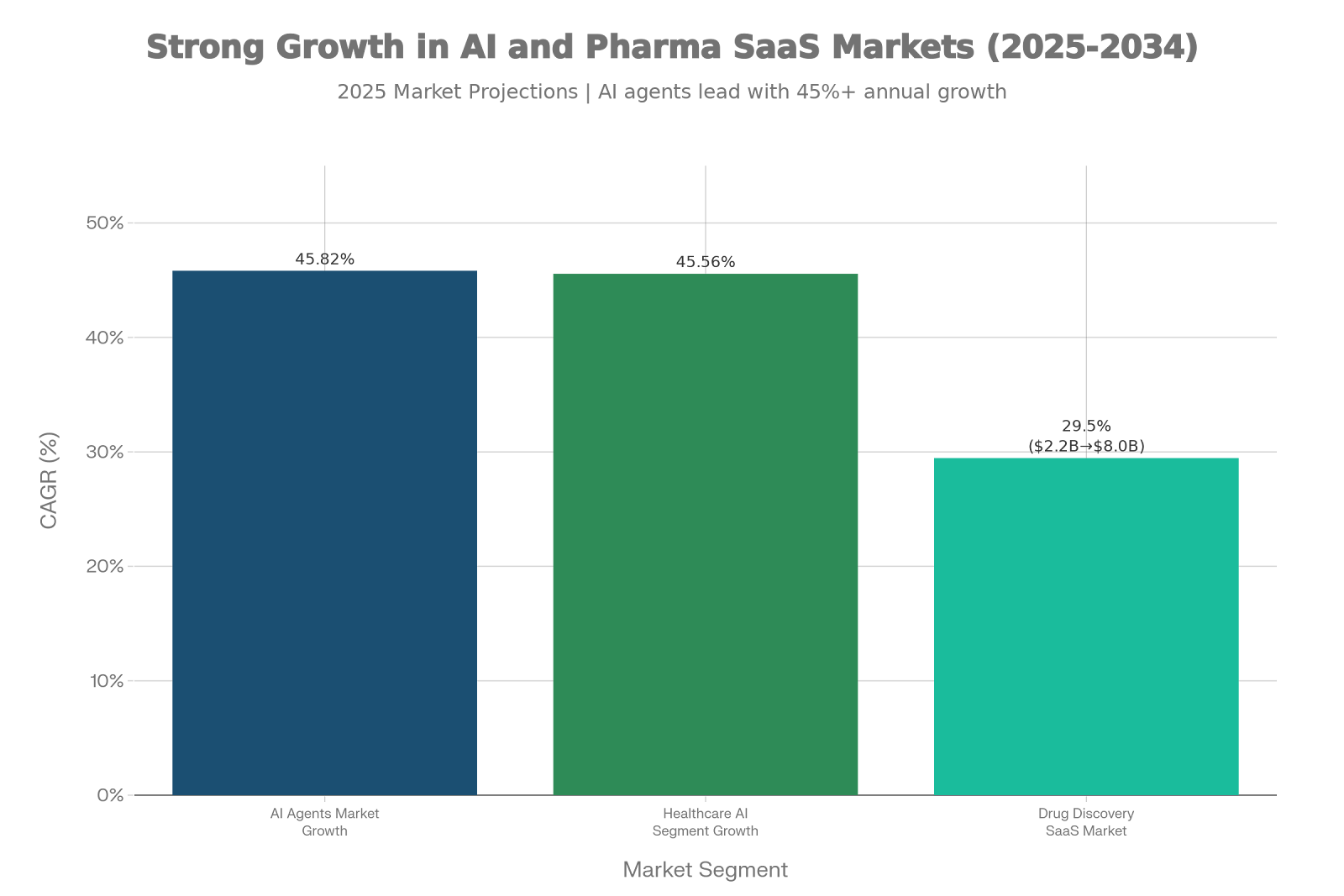
Market Growth: AI Agents and Pharmaceutical SaaS Expansion (2025-2034)
Within life sciences specifically, agentic AI is growing even faster at 45.56% CAGR, driven by the convergence of three four powerful trends:
- Regulatory Complexity is Accelerating: FDA, EMA, and global health authorities are tightening requirements around documentation, traceability, and real-time compliance monitoring. Teams need systems that can work 24/7 to monitor regulatory changes and alert stakeholders instantly.
- Data Volumes are Exploding: Medical inquiries, adverse event reports, literature, social media signals, and claims data arrive from dozens of sources simultaneously. Manual triage is no longer feasible. Pharmaceutical companies that adopt AI-driven signal detection are reporting up to 40% increases in identified adverse events by incorporating real-world data sources.
- Operational Efficiency is Table Stakes: Leading pharma companies have already begun their AI transformation. Those investing in medical affairs automation today are seeing 30-40% reductions in response times, significant decreases in data entry errors, and freed-up teams who can focus on strategic, high-value scientific interactions rather than routine administrative tasks.
- Expansion in Direct-to Consumer Marketing (DTC): As pharmaceutical companies continue to expand direct-to-consumer (DTC) marketing, industry forecasts project sustained growth of approximately 4.0–5.6% CAGR over the next decade, with digital DTC outreach growing even faster at ~10% CAGR. This increased visibility and accessibility of branded and disease-state messaging is driving higher engagement from both patients and healthcare professionals (HCPs), resulting in a significant rise in volume, velocity, and complexity of unsolicited medical inquiries, outpacing the capacity of traditional Medical Affairs teams. To efficiently deliver scientifically accurate, compliant, and timely responses, an AI-driven Medical Affairs solution is no longer optional but a strategic imperative.
SCIMAX ARIN: Expanding Intelligence Across Medical Affairs
Our vision for ARIN is clear: purpose-built intelligence that strengthens not replace the scientific judgement of Medical Affairs teams. The Fall 2025 expansion deepens ARIN’s footprint across inquiry management, safety signal detection, and scientific communications.
Refer to our page https://scimaxglobal.com/medicalaffairsaiagents/scimax-arin/ for all our agent’s information.
We focus our agent’s categorization on aligning with Med Affairs teams key focus areas:
Scientific, Operational, and Cross-Functional
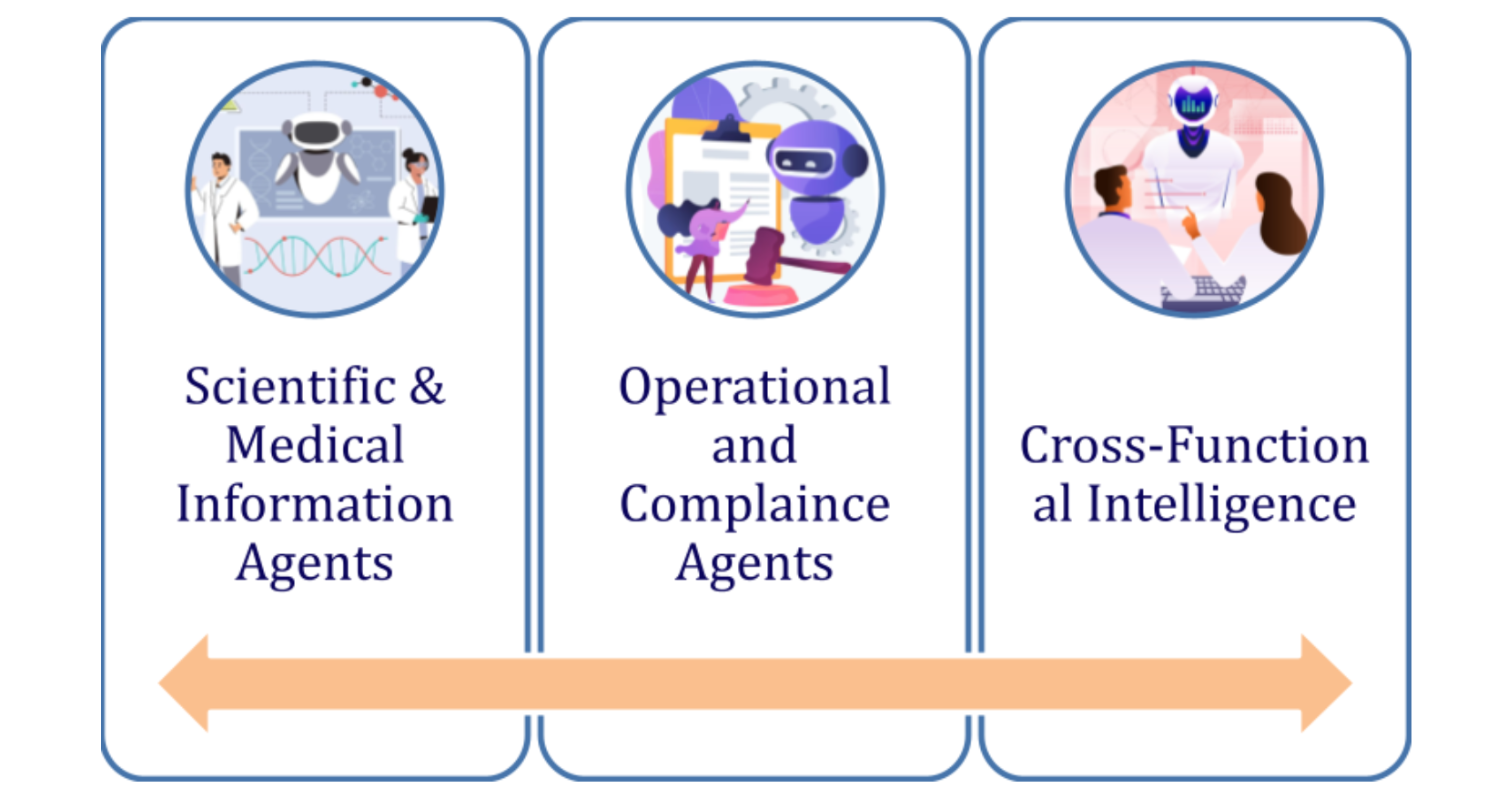
Three Categories of AI Agents: Scientific, Operational, and Cross-Functional
ARIN Platform Roadmap: A Client-Centric Approach to Medical Affairs AI
At its core, our product philosophy is straightforward: identify real-world use cases, develop solutions with rigor, gather client feedback early, and refine relentlessly before production release. This isn’t a theoretical exercise or a technology-first approach. This is a pragmatic, market-driven methodology designed to ensure every agent we release solves genuine problems that medical affairs teams face every single day.
Our product roadmap begins with intensive engagement with medical affairs leaders, compliance officers, pharmacovigilance directors, and MSLs across our clients and prospects. We listen to the specific bottlenecks they encounter. We understand the regulatory constraints they navigate and the operational pressures they face. This discovery process is continuous, not a one-time exercise. The 10 agents we’ve identified represent the highest-impact opportunities we’ve identified through this rigorous, evidence-based discovery process.
Our development team builds each agent with extraordinary rigor. We design for compliance from day one integrating regulatory standards and expectations directly into architecture.
The Early-Access Phase: Real Clients, Real Workflows, Real Feedback is our methodology differentiates us most fundamentally. Rather than releasing agents into the wild and hoping they work, we deploy them into sandbox environments with select early-access clients. What we learn in the sandbox phase is invaluable. We monitor agent performance in real time, flag accuracy drift immediately, and prioritize refinements based on client priorities.
Only after an agent has proven itself in sandbox environments with real clients, real data, real regulatory oversight, do we graduate it to general availability. This means when we deploy these agents into production environment, they’re not experiments. They’re battle-tested solutions.
As we continue expanding our agent portfolio throughout 2025-2026, this development methodology ensures you benefit from a continuous stream of validated, client-tested innovationsinnovations.
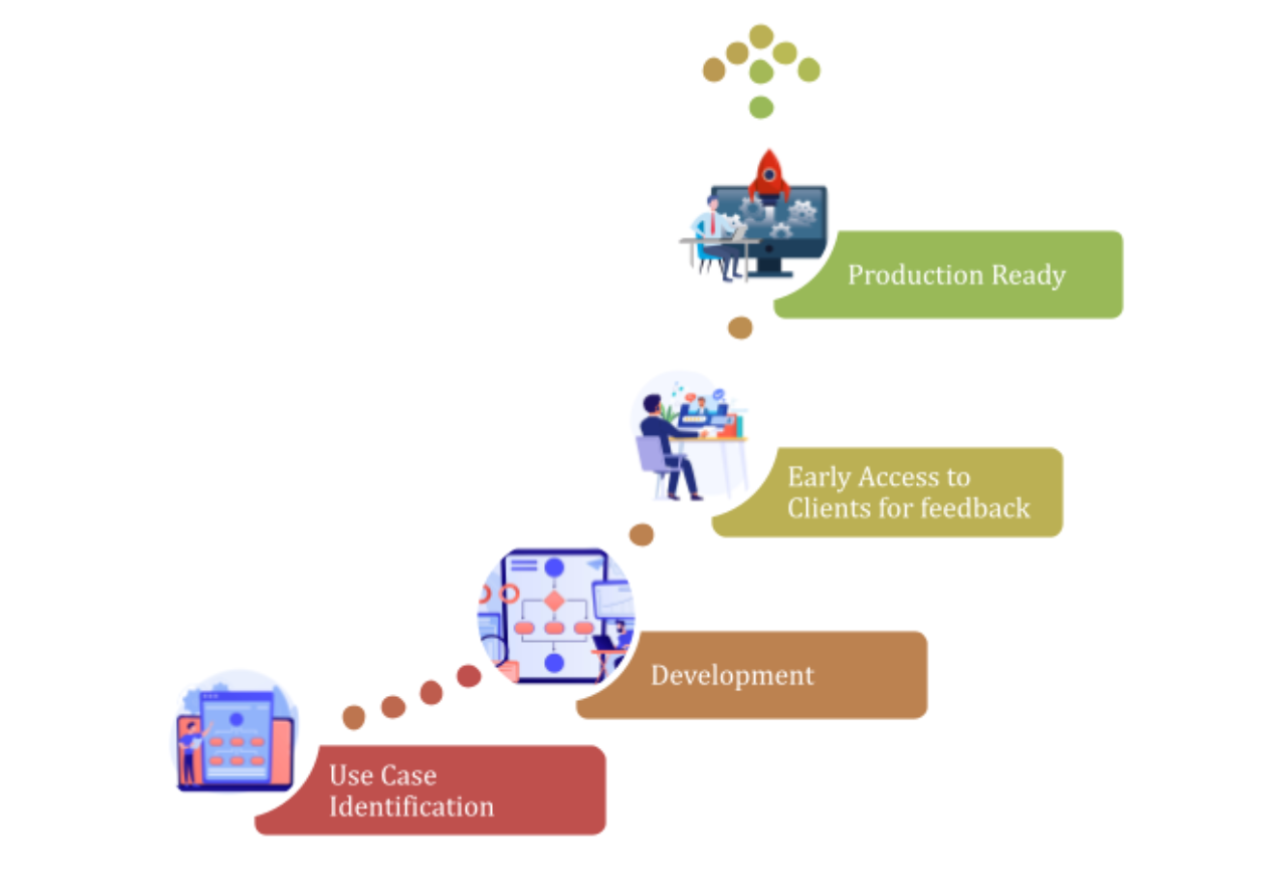
ARIN Agents Development Roadmap: From Identification to Market Release
As CPO, my commitment is to build technology that strengthens scientific leadership, not just operational efficiency. SciMax remains focused on delivering a platform that supports the future of Medical Affairs.
— Saad Rahman
Chief Product Officer, SciMax Global
References:
- https://meditechinsights.com/agentic-ai-in-healthcare-market/
- https://www.strategicmarketresearch.com/market-report/drug-discovery-saas-platforms-market
- https://www.wiseguyreports.com/reports/direct-to-consumer-pharmaceutical-advertising-market?utm_source=chatgpt.com
- https://gitnux.org/marketing-in-the-pharma-industry-statistics/?utm_source=chatgpt.com

