From Planning to Launch in 10 Weeks: Implementing a Validated Medical Information Solution for a Novel Drug-Device Product
Overview
Many emerging biopharma companies operate with limited Medical Affairs resources, and Medical Information is often the most affected function. As organizations focus on progressing R&D and securing regulatory approvals, digital investment in Medical Information may fall behind, leaving teams without a structured Medical Information Management System to support compliant, consistent communications.
As a core component of Medical Affairs, Medical Information is essential for ensuring timely, accurate, and regulatory-compliant scientific communication to healthcare providers, patients, and caregivers.
Situation
SQ Innovation is a privately held biopharmaceutical company in Burlington, Massachusetts. The company was established to develop and commercialize innovative, cost-effective therapies that enable subcutaneous delivery of pharmaceutical products where and when they are needed. Its first product was developed specifically for the treatment of fluid overload due to worsening heart failure.
SQ Innovation entered its commercialization phase with a Medical Information model already established through its partnership with a contracted Medical Information service provider. As part of its inaugural product launch planning, the company further evaluated multiple integration approaches — including leveraging its contracted Medical Information service provider’s instance of SCIMAX MI. Ultimately, SQ Innovation made the strategic decision to implement its own validated instance of a Medical Information Management System.
The goal was to ensure accurate, balanced, non-promotional, and compliant medical product information for healthcare providers, patients, and caregivers, while delivering a high-quality inquiry experience.
The Challenge
After electing to implement and maintain its own dedicated instance of the SCIMAX MI platform, SQ Innovation focused on preparing Medical Information operations to be fully integrated, compliant, and launch ready. This required coordinated cross-functional effort across Medical Information, Complaint Management, Pharmacovigilance, and Technology Program Management to:
- Gain full proficiency through platform training for internal teams.
- Define and implement a configuration aligned with SQ Innovation’s novel drug-device product.
- Integrate the MI platform with complaint management, order fulfillment and other systems.
- Establish the processes and workflows necessary to support inquiry handling and system interoperability.
- Onboard the contracted Medical Information service provider partner to SQ Innovation’s configured instance.
The Solution
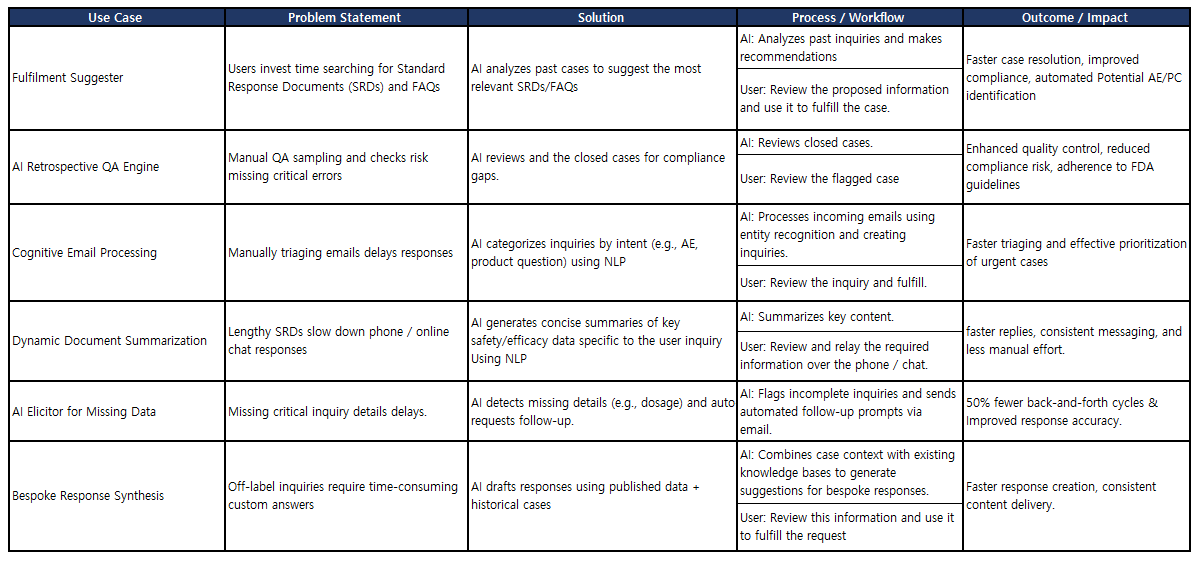
SciMax Global implemented the SCIMAX MI Solution to centralize and automate SQ Innovation’s medical information management, ensuring timely configuration, validation, and ‘go-live’ within 10 weeks. Meeting these requirements necessitated a detailed project plan and strong project management oversight, which SciMax Global delivered alongside the SCIMAX MI technological solution.
The SCIMAX MI Solution is a cloud-based medical information management platform built for the life science industry.
Key solution elements include:
- SCIMAX MI (Medical Information): Centralizes intake, triage, fulfilment, and analytics across all MI, AE, & Product Complaint workflows. Configurable case form, multilingual content, and standardised correspondence minimize response times while adhering to the governance requirements.
- A secure four-tier cloud infrastructure is deployed for Development, Validation, Production, and Disaster Recovery environments to provide resilience, compliance, and uninterrupted operations.
- Configurable workflows for medical inquiries and product compliance to streamline case handling.
- Automated routing and tracking, reducing manual entry and improving response turnaround time.
- Built-in reporting and dashboards for real-time visibility into inquiry volumes and SLA performance.
- Comprehensive system validation aligned with FDA 21 CFR Part 11, GxP, and GDPR requirements.
- A structured enablement approach, including a train-the-trainer model, empowering internal teams for long-term system sustainability, supported by extensive training and change management control.
The Impact
Implementing their own dedicated instance of SCIMAX MI allowed SQ Innovation to maintain full configuration control, ensure data stewardship and support its novel drug-device product, ultimately enabling SQ Innovation to deliver a higher quality customer experience.
SciMax Global supported SQ Innovation in achieving the speed, consistency, and audit readiness required for launch. These benefits were reflected in measurable improvements across efficiency, compliance, and user experience, including:
- Accelerated case processing through streamlined workflows.
- Enhanced compliance oversight with full traceability and validated audit trails.
- Fully digital medical information and email management, reducing paper-based processes.
- Real-time reporting and analytics that empower data-driven decision-making.
Outcome
The collaboration between SQ Innovation and SciMax Global exemplifies how strategic IT partnership and deep Medical Information and Medical Affairs expertise resulted in a robust, compliant, and future-ready Medical Information Management System for commercialization. With SCIMAX MI, life sciences organizations can confidently manage medical information in a centralized, validated, and automated environment that drives efficiency, compliance, and operational excellence.
“Delivering a fully compliant medical information platform for SQ Innovation in just 10 weeks demonstrates what we stand for at SciMax – speed, rigor, and strategic impact. Medical information systems should be catalysts for growth, not constraints. We build with that conviction every time.”
– Saad Rahman, Chief Product Officer, SciMax Global.
“Our goal was to ensure that every aspect of Medical Information – from configuration to integrations to inquiry experience – was fully aligned with our product and operations. SciMax Global’s SCIMAX MI Solution and structured approach supported us in achieving that level of readiness.”
– Bryn Taudvin, Director, Medical Information/Pharmacovigilance, SQ Innovation, Inc.