How artificial intelligence can cut down turnaround time for Medical Query
Delays in responding to inquiries from healthcare professionals, Care Givers, Health Authority and Patients globally creates an operational drag on the Medical Affairs, Medical Information and Regulatory Teams of an organisation. The stakes are very high; extended time taken while responding erode HCP confidence and impact adversely on submission timelines during NDA/BLA reviews.
Purposeful implementation of AI in Medical communication can reduce the time taken in responding to an inquiry from days to hours, and maintaining auditability, consistent with ability to globally scale.
Operational chokepoints in pharma/biotech communications:
- Fragmented intake and handoffs: There are various intake channels (email, phone, portals and fields agents) leading to redundancy and delay in inquiry triaging.
- Compliance complexity: Global medical communication team is contingent upon stringent version control, audit trail, HIPAA, GDPR, GxP and FDA 21 CFR Part 11 compliance.
- Content Sprawl: The identification of appropriate approved response set as per the product, indication and region criteria must be performed by the responder while promptly rising off-label or safety signals.
- Regulatory pressure: Health Authority investigation during the NDA/BLA review requires brief, cross functional responses under tighter timelines.
How AI simplifies handling medical inquiries
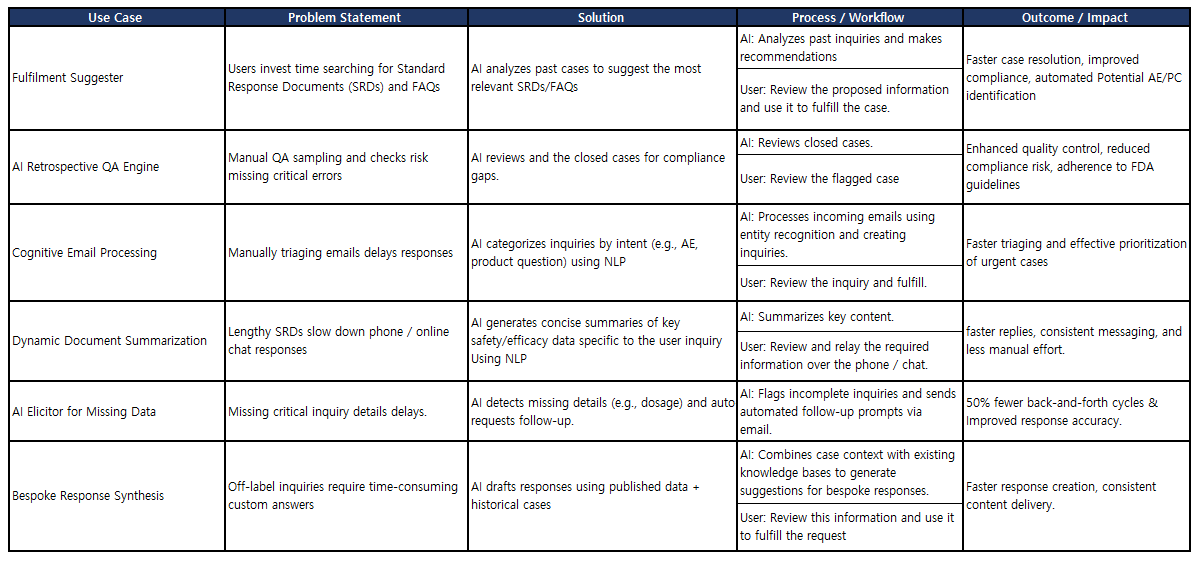
- Automated intake and triage: AI agents extract intent, identify requestor type (HCP, Patient, Caregiver), discover safety or product complaint and based on the identified information route case to right queues, prevent backlog, while ensuring urgent category (AE/PC) are prioritised.
- NLP for classification and drafting: Natural language models align inquires to the approved content, extract country specific standard response documents and FAQs and build a response package with appropriate enclosures and cover letters.
- Priority scoring and escalations: Policy guided rules and AI backed signalling identify off-label queries, escalate to Medical Directors or Safety within seconds, and create a dependable trail of decisions.
- Content access and governance: System accesses the usage of latest approved content, enforces consistency, and keep track of who used what, when and why.
- Accelerate collaboration: Use AI to assist cross-functional teams (Regulatory, Medical Affairs, Safety, Legal) in summarizing previous correspondence, keeping track of commitments and proposing what next-step activities are needed with deadlines.
- Reporting and forecasting: The real time dashboards focus on bottlenecks, SLA risk and high priority concerns; sematic analysis to detect new themes (e.g. adverse events alert) so content and safety responses can be updated ahead of schedule.
SCIMAX: purpose build to realize the benefits
- SCIMAX MI (Medical Information): Centralizes intake, triage, fulfilment, and analytics across all MI, AE, & Product Complaint workflows. Configurable case form, multilingual content, and standardised correspondence minimize response times while adhering to the governance requirements.
- SCIMAX CP (Collaboration Portal): Offers a branded, multilingual front door for HCPs & Consumers with profile specific experiences, searchable approved content and self-service access to MI documents lower inbound case volume whilst increasing satisfaction.
- SCIMAX ARIN (AI agents): AI driven triage, NLP classification, response drafting and escalation detection which can plug directly into the SCIMAX MI and CP. ARIN accelerates velocity and compliance by setting up rules for automation of the “first mile” of case management and covering the “last mile” as response development.
Real-world use cases towards reduced resolution times
- Global multilingual HCP questions: A Cardiologist in Germany is looking for off label mechanism detail. ARIN identifies off label intent, highlights to escalation and forwards to Medical Director where as SCIMAX MI pre-calibrates the approved, country specific response set. The result: rapid and compliant reply with full context and audit metadata.
- Proactive off label detection: A group of related fields directed question suggests possibly off label use. ARIN’s semantic grouping identifies the trend, informs Medical Affairs, and aid in updating the standard response package and FAQs.
- Adverse events and product complaints: AI classifies and annotate AE/PC terminology and information is transmitted to the Safety system based on the auto-transmission rules. E2B compliant payload reduces need of manual rekeying and time to notify in a secure way without compromising data integrity.
- Health Authority during NDA/BLA: Receives numerous overlapping queries across CMC, clinical and labelling. ARIN consolidates dossier evidence, maps citations to up-to-date controlled document and generates structured responses for expert sign-off reducing cycles from weeks to days whilst safeguarding submission schedules.
- Medical publication support: AI fast-tracks literature triage, retrieved controlled language from response package and establish links to the published version shrinking the time to provide evidence-based response while preserving version identification.
Built into the workflow
- HIPAA and GDPR compliance: Access controls, data encryption in transit and at rest, data minimization during intake and fulfilment safeguard personal information.
- GxP and 21 CFR Part 11: A combination of electronic records, validated systems and change controls provide data integrity and traceability.
- Versioning control and audit trail: Every step from inquiry intake, classification, content selection, response packaging, review, provides an absolute, timestamped record, ensuring audit readiness.
- Role-based access and least privilege: Medical Information, Safety, Regulatory, and Legal see only what they should (I.e., workflows with adequate review and approval)
Measurable value
- Speed and consistency: Shrink response time by employing automated AI-assisted intake, classification and content assembly, all while ensuring one source of truth content and labelling.
- Compliance confidence: Validated systems, audit trails and version control minimize inspection risk and enhance documentation quality throughout MI, Safety & Regulatory.
- Reduction in submission cycle time: AI-enabled orchestration of Health Authority queries reduces response turnarounds and shields NDA/BLA review timelines.
- Publications and evidence alignment: Faster access to controlled scientific terms and references enhances response quality while reducing redundancy.
- Resource Optimization: Specialist can shift their focus on complex scientific and regulatory task as the routine cases are automated, initial draft response created with the help AI.
What Good looks like with SCIMAX
- Intake to triage in minutes: ARIN identifies intent, language and sensitivity; SCIMAX MI routes it into the right queue with the right priority.
- Response packaging in hours: Assembling of initial response with the cover letter and country specific approved content selection and references available for the reviewer.
- Frictionless safety submissions: AE/PC activates auto push to safety systems with mandatory fields, acknowledgement and confirmation records.
- Submission-grade traceability: Every step is traceable with effective use of electronic signatures, date/timestamps, and version links in compliance to 21 CFR Part 11 and GxP.
- Iterative improvement: SLA performance, inquiry trends and knowledge gaps are provided in reports/dashboards enabling proactive maintenance for Standard response and FAQs.
Conclusion and takeaways
AI in medical communication is no longer a nice to have feature. It unlocks the true potential by reducing the medical query turnaround time, safeguarding compliance, and protecting regulatory timelines. Multi-channel automated case intake, triage leveraging NLP, rule base content assembly and response packaging, self-service portal equips medical affairs, medical information and regulatory team to deliver faster, consistent communication with scale.
Learn how SCIMAX MI and SCIMAX ARIN transform medical affairs operation, while SCIMAX CP provide a secure, global self-service for HCPs and consumers.
Contact us for a tailored demonstration.
Author
Sainath Racherla
Associate Director – Program Management

